National Cancer Institute violates law, lets cancer patients down
- Till Bruckner
- Apr 18, 2018
- 2 min read
The National Cancer Institute is failing to post the results of its clinical trials on Clinicaltrials.gov, slowing down the search for new treaments and cures for cancer, data compiled by the EBM Data Lab shows.
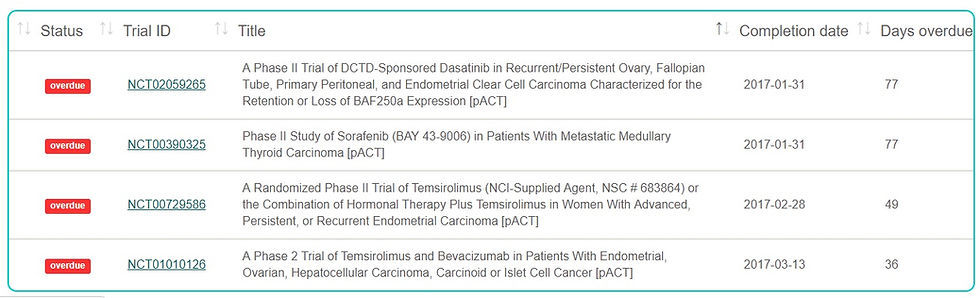
According to the lab's FDAAA TrialsTracker, which monitors whether trial sponsors comply with their legal - and ethical - requirement to post clinical trial results within 12 months of trial completion, the NCI has failed to post the results of four clinical trials over the past months. It posted the results for two further trials only after the deadline set by law had already expired.
In total, out of ten clinical trials sponsored by the NCI, six violated the FDA Amendment Act, a key transparency law. Under that law, the NCI has already run up fines of over $1.5 million - but the FDA has so far failed to collect a single cent in fines.

Cancer patients suffering from Clear Cell Carcinoma, Metastatic Medullary Thyroid Carcinoma, and Endometrial Carcinoma participated in the four unreported NCI trials.
Patients volunteer to participate in clinical trials in the hope that they will contribute to the development of new treatments. However, if trial results are not widely shared, scientists cannot build on each others' work. The NCI's failure to post trial results thus violates the trust placed in it by cancer patients across the United States.
This blog will track the NCI's clinical trial transparency performance as more trials become due to post results over the coming months.
To learn more about the harm caused by not posting the summary results of clinical trials, and the FDAAA, read this study by Transparency International, TranspariMED, Cochrane and CRIT.

Comments