Austria: 273 clinical trials of medicines are missing results
- Till Bruckner
- Feb 24, 2020
- 3 min read
Austrian universities, hospitals and pharma companies have failed to upload the results of at least 273 clinical trials of medicines onto the European trial registry, a study released today by TranspariMED, Cochrane Austria and Transparency International Austria shows.
In total, 82% of due trial results are missing in Austria.
UPDATE: Following strong media coverage of TranspariMED's report, a group of Austrian parliamentarians wrote a letter to the Minister of Health asking him what the ministry is doing to improve reporting rates, and whether and when he plans to introduce sanctions for universities that fail to make trial results public.
Institutions’ failure to comply with European Union transparency rules means that information on the safety and effectiveness of medicines gained through research involving Austrian patients is often made public only with long delays, or not at all. Most of this research was conducted by public institutions financed with taxpayers’ money.
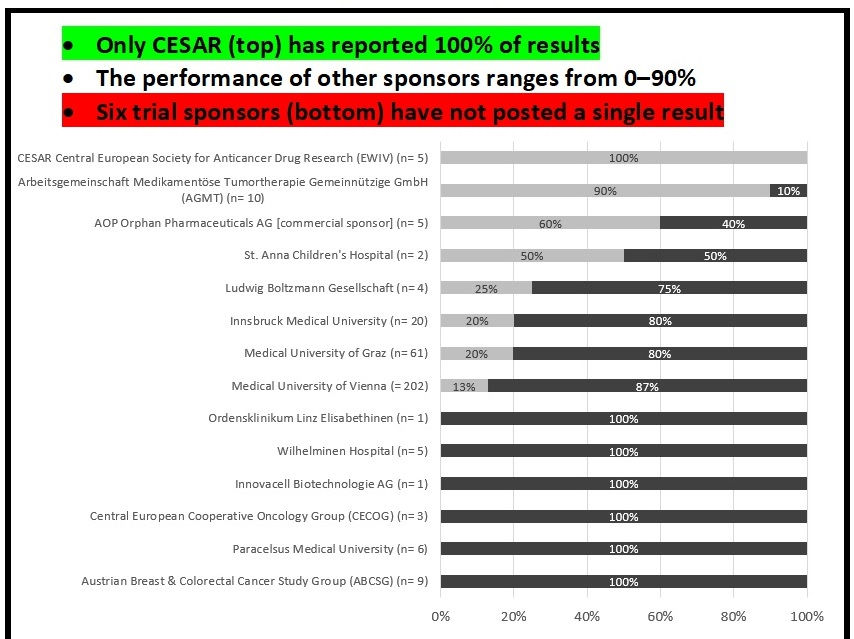
Only one major institution, the Central European Society for Anticancer Drug Research (CESAR) has a perfect 100% reporting rate. The performance of other sponsors ranges widely, from 0–90%. Six out of the 14 largest trial sponsors in Austria have not posted a single result. The country’s largest medical research institution, Vienna Medical University, has only posted 13% of trial results.
With 176 due trials missing results, the Medical University of Vienna has by far the largest backlog of unreported trials, followed by the universities of Graz and Innsbruck.
Overall, medical research institutions in Austria perform better than those in Germany, but are still far behind those in Ireland and the UK.
On the positive side, the three largest universities are already working to fix the problem.
Vienna and Graz uploaded more results over the past year than during all the previous years combined, and Innsbruck has stated that it will increase its uploading of trial results.
Reacting to the study, Medical University Vienna told broadcaster ORF that the university will continue its efforts to upload more results, and is already in the process of developing a guide for its researchers with tips and tricks for uploading results onto the registry. In addition, the university will identify registry entries with incorrect and out-of-date data, and send out reminders to the researchers who ran them, together with offers of support for data entry.
Austria’s national regulator, the Austrian Federal Office for Safety in Health Care (BASG), welcomed the report.
In a written statement, BASG noted that:
“[BASG] supports the importance of completeness and accuracy of data on trials and trial results… BASG is in constant and close collaboration with these sponsors and their respective Ethics Committees to increase data quality and completeness in EudraCT… BASG is definitely seeing an improvement in data quality and completeness in EudraCT over time.”
The regulator also endorsed the report’s call for stronger monitoring of compliance by the Austrian government:
“BASG considers the proposals made in the publication as appropriate… BASG has already communicated the issue of compliance to result reporting to the Ministry of Health and will continue to do so in order to support changes of the legal framework.”
Andrea Fried of Transparency International Austria said:
“Weak compliance means that important research results are being kept from the public. That not only violates rules around scientific integrity, but is also irresponsible towards the patients who participated in these studies. Furthermore, public money is being wasted.”
Gerald Gartlehner, chair of Cochrane Austria, said:
“Of course, this raises the suspicion that results are not being made public because these studies do not show the results that their funders were hoping for. Further down the road, the absence of results can lead to patients being treated with therapies that lack effectiveness, because doctors lack this information.”
Till Bruckner, founder of TranspariMED, said:
“It is encouraging that the largest Austrian universities and BASG are already aware of the problem and are working to fix it. However, international experience shows that full compliance cannot achieved without sanctions. The Austrian government should follow the example of the UK government and set up a national monitoring system covering all clinical trials conducted within the country.”
The full study is available online. Universities in Austria and other European countries can use TranspariMED’s collection of transparency tools to improve their clinical trial reporting.


Comments