top of page

Working to end evidence distortion in medicine
.webp)
The Full Story
TranspariMED campaigns for all clinical trial results to be rapidly made public.
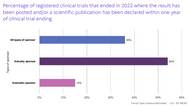
Many clinical trial results are never made public, leaving dangerous gaps in our understanding of how safe and effective medicines, medical devices and other treatments really are. This harms patients, undermines public health, and wastes public money.
We have already saved thousands of clinical trial results from becoming forever lost:
Many pharma companies and universities in Europe and the United States have stopped ignoring transparency laws and started publishing missing results
Many funding institutions worldwide now check whether their researchers publish all trial results
The United Kingdom has adopted the world’s first national transparency strategy
Recent News
Archive
Featured Posts
bottom of page